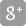Overview
Typical activities
- Need identification
- Customer complaints
- Customer/user/patient discussions and collaborations
- Academic research collaboration
- Funding opportunities
- Reimbursement strategy
- Business plan
- Health economics
- Market research/opportunity, competitve analysis
- Immaterial property evaluation or filing
- Early risk assessement
- Literature reviews
Support functions
- Incubators
- Investors
- Umbrella organisations
- Lobbying
- Education
- Collaborative programs
Typical activities
- Customer needs identified and verified
- Customer needs transferred into requirements
- Usability requirements
- Preliminary device classification (MDD, IVDD, AIMDD)
Develop product specifications; needs and demands of both customers and subcontractors
Typical activities
- Unambiguous requirements possible to verify
- Functional, performance, interface requirements
- Safety, regulatory requirements
- Packing, labeling, installation requirements
- Preliminary risk analysis
- Formative usability testing
- Quality management system implemented
Typical activities
- Drawings, material specifications, software code, assembly instructions etc.
- Continued risk analysis
- IP strategy
- Continued formative usability testing
Verification of components and processes in relevant environment.
Evaluation that the design meets the requirements
Evaluation that the design meets the requirements
Typical activities
- Confirmation that all product requirements are fulfilled
- May include testing of biocompatibility, EMC, transportation and storage
Prototype resembles final product in function and form.
Evaluation of your design under controlled circumstances; clinical evaluations.
Evaluation of your design under controlled circumstances; clinical evaluations.
Typical activities
- Confirmation that the design conforms with the intended use and with user needs
- Testing as close to real use conditions as possible
- May include clinical studies
- Summative usability testing
- Clinical evaluation
- Final risk management report
Typical activities
- Packing, labeling
- Manufacturing quality plan, 'Device master Record',
'Device History Record' - Supply chain
- Distribution
Typical activities
- Competent authority / Notified body
- FDA and national regualtions
- Premarket application or notification submitted or approved
Product in its final form ready for full commercial deployment in relevant operating environment
Typical activities
- Sales and marketing
- Post-market clinical follow-up sudies
- Customer complaints handling
- Vigilance handling
- Continued risk management
- Usability surveillance
Upcoming Life Sciences Events
- April 2024
- Basel: Swiss Biotech Day 2024
- London: BioTrinity 2024
- Singapore: Asia Bio Partnering Forum
 Biotechgate Global Database ›
Biotechgate Global Database ›